For a long time, different materials had been known to burn with different colors. When scientists realized that prisms could take a beam of light and spread it out like a rainbow that allowed the constituent colors to be seen (a spectrum), they realized that pure materials (elements) each had their own pattern of discrete colors (wavelengths). One of the greatest puzzles in Physics was why this was the case? Why couldn’t an element like hydrogen, for example, emit any wavelength?
On the otherhand, if hydrogen was placed between a lightsource and a prism, the resulting spectrum still showed the hydrogen spectrum – but reversed! The lines were now black!
To explain why elements like hydrogen only produce discrete spectra, that are sometimes bright (emission spectra) and sometimes dark (absorption spectra) Neils Bohr proposed the electron in the hydrogen atom could only exist at particular distances (orbits) from the nucleus. If an electron gained energy it could move up to a higher orbit – but only if it gained exactly the right amount of energy corresponding to the difference in orbit energies. It might gain energy from atoms bumping into each other, or by absorbing a photon with that exact energy; absorption spectra are produced only when photons are absorbed – specific energy photons are removed from the incoming light source.
Similarly, an electron can jump to a lower orbit if it emits a photon with exactly the energy corresponding to the energy difference between the final and initial orbits; this is what causes emission spectra. Because different atoms have different numbers of protons and electrons, the orbits are not quite the same and are unique for each atom. Therefore, different atoms will have different spectra which act like fingerprints allowing us to identify them.
Your goal is to explore how spectra are created as photons are absorbed and emitted by electrons.
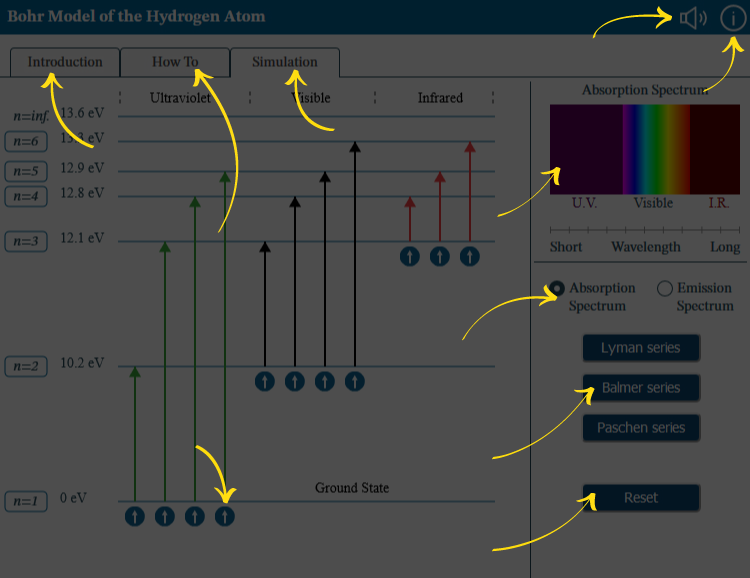
First, in the controls region, choose whether to explore the absorption or emission spectrum. Optionally, you can restrict your explorations to the Paschen, Balmer, or Lyman series. Reset the simulation at any time to clear the spectrum and remove series restrictions.
Next, familiarize yourself with the default state of that spectrum and with the information about the energy levels associated with each possible electron orbit. If you choose the absorption spectrum you will see that all the electrons start at low orbits and have arrows pointing toward higher ones. If you choose the emission spectrum, you will see the opposite: all the electrons start at high orbits and have arrows pointing toward lower ones.
Use any of the round “electron” buttons in the main “simulation” region to cause a photon to be absorbed or emitted by an electron in this Hydrogen atom. Different buttons will cause photons of different wavelengths to be absorbed or emitted. As the photon is absorbed or emitted, its color and wavelength will be revealed. The absorption or emission spectrum you examined before will also update.
If the sound is on, you will hear sounds to indicate:
- When the electron absorbs or emits a photon.
- When the electron is traveling to a higher or lower orbit.
- Each time the electron reaches or passes an orbit-threshold.
Your goal is to explore how spectra are created as photons are absorbed and emitted by electrons. This simulation is divided into 5 regions:
- Title banner with the audio on/off and info buttons.
- Navigation options.
- Controls region where you can choose your spectrum type and limit your spectrum to a particular series.
- Main content region where you activate each electron to absorb or emit a photon.
- Spectrum region, which shows the current state of the spectrum you create.
Visit the How To tab for details.
Ultraviolet only. Activate to disable photons that impact visible and infrared light.
Visible light only. Activate to disable photons that impact ultraviolet and infrared light.
Infrared only. Activate to disable photons that impact ultraviolet and visible light.
Clear spectral lines and series selection.
Moves from n=1, the ground state, to n=2. 0 eV to 10.2 eV. Absorbed photon is ultraviolet with wavelength=122 nm. In absorption spectrum, a thin line of ultraviolet light, far from visible spectrum, is no longer visible.
Moves from n=1, the ground state, to n=3. 0 eV to 12.1 eV. Absorbed photon is ultraviolet with wavelength=103 nm. In absorption spectrum, a thin line of ultraviolet light is no longer visible; this line is very slightly closer to the visible spectrum than that from the jump from n=1 to n=2.
Moves from n=1, the ground state, to n=4. 0 eV to 12.8 eV. Absorbed photon is ultraviolet with wavelength=97 nm. In absorption spectrum, a thin line of ultraviolet light is no longer visible; this line is very slightly closer to the visible spectrum than that from the jump from n=1 to n=3.
Moves from n=1, the ground state, to n=5. 0 eV to 12.9 eV. Absorbed photon is ultraviolet with wavelength=95 nm. In absorption spectrum, a thin line of ultraviolet light is no longer visible; this line is very slightly closer to the visible spectrum than that from the jump from n=1 to n=4.
Moves from n=2 to n=3. 10.2 eV to 12.1 eV. Absorbed photon is red visible light with wavelength=656 nm. Absorption spectrum shows a thin line of red light is no longer visible.
Moves from n=2 to n=4. 10.2 eV to 12.8 eV. Absorbed photon is blue visible light with wavelength=486 nm. In absorption spectrum, a thin line of blue light is no longer visible.
Moves from n=2 to n=5. 10.2 eV to 12.9 eV. Absorbed photon is indigo visible light with wavelength=434 nm. In absorption spectrum, a thin line of indigo light is no longer visible.
Moves from n=2 to n=6. 10.2 eV to 13.3 eV. Absorbed photon is violet visible light with wavelength=410 nm. In absorption spectrum, a thin line of violet light is no longer visible.
Moves from n=3 to n=4. 12.1 eV to 12.8 eV. Absorbed photon is infrared with wavelength=1875 nm. Wavelength is too long to appear on the absorption spectrum chart shown in this interactive.
Moves from n=3 to n=5. 12.1 eV to 12.9 eV. Absorbed photon is infrared with wavelength=1282 nm. Wavelength is too long to appear on the absorption spectrum chart shown in this interactive.
Moves from n=3 to n=6. 12.1 eV to 13.3 eV. Absorbed photon is infrared with wavelength=1094 nm. Wavelength is too long to appear on the absorption spectrum chart shown in this interactive.
Default absorption spectrum, from short to long wavelengths, with a thick band of ultraviolet, a similar-sized band of visible light, and a thinner band of infrared. The visible band contains:
- Thin band of violet.
- Medium band of indigo.
- Thin band of blue.
- Medium band of green.
- Thin band of yellow.
- Medium-thin band of orange.
- Medium-thin band of red.
Audio: Turn sounds off or on. See How To tab for details on what the sounds indicate.
Information: Reopen this overview screen.
Introduction tab contains background information about the subject of the simulation.
How To tab contains detailed information about how to use the simulation.
Simulation tab contains the simulation.
Spectrum Type: Choose either the Absorption or Emission Spectrum.
Choose a series to see the spectrum that results from that subset of electrons.
Use Reset to clear the spectrum of all lines and start again.
Use the electron buttons to cause hydrogen to absorb or emit the photon.
Absorption or Emission Spectrum: Observe how wavelengths appear as photons are absorbed or emitted.